Impella Connect Achieves Regulatory Milestone
Business Wire 31-Jan-2019 8:00 AM
Cloud-based monitoring platform gains CE Mark, adding to FDA PMA
approval
Abiomed
(NASDAQ:ABMD) has achieved CE Mark for Impella Connect, the first-of-its
kind cloud-based technology that enables secure, real-time, remote
viewing of the Impella console for physicians and hospital staff from
anywhere with Internet connectivity. European CE Mark adds to Impella
Connect's previous U.S. FDA PMA approval.
This press release features multimedia. View the full release here:
https://www.businesswire.com/news/home/20190131005297/en/
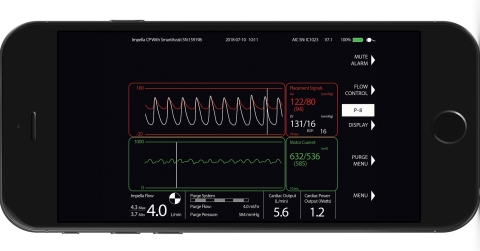
Impella Connect allows medical professionals to view the Impella console, in real-time, from any Internet-connected computer or mobile device. (Photo: Abiomed, Inc.)
Impella Connect uses real-time intelligence to help improve patient
outcomes. In addition to allowing medical professionals to view their
hospital's consoles remotely, Impella Connect allows highly-trained
staff at Abiomed's 24x7 Clinical Support Center to provide medical
professionals with expert evaluation of Impella data and real-time
collaborative patient management.
"Impella Connect is an extremely valuable resource that allows me, as
well as allied health professionals and nursing staff, to have direct
visualization of data from the Impella console and to closely monitor
patients on hemodynamic support, in real time," said Rajeev L. Narayan,
MD, Assistant Director of Structural Heart Intervention and Director
Interventional Mechanical Circulatory Support, Vassar Brothers Medical
Center.
Based on the previous FDA PMA approval, Impella Connect, which is fully
HIPAA compliant, is in a limited market release in the United States. 36
hospital sites are currently using the technology on a regular basis to
provide enhanced real-time support for their patients. Abiomed will
launch Impella Connect in Europe this quarter through a controlled
roll-out at hospital sites with established heart recovery protocols.
The first hospital will be University Heart Center in Hamburg, Germany.
"Impella Connect is a technological advancement which represents the
next frontier of heart recovery products," said Michael R. Minogue,
President, Chairman and CEO of Abiomed. "Impella Connect, along with our
24x7 onsite and on-call support, enables physicians, nurses and ICU
staff to increase productivity, improve patient outcomes, and help
patients return home with their native heart."
ABOUT IMPELLA HEART PUMPS
The Impella 2.5 and Impella CP devices are FDA approved to treat certain
advanced heart failure patients undergoing elective and urgent
percutaneous coronary interventions (PCI) such as stenting or balloon
angioplasty, to re-open blocked coronary arteries. The Impella 2.5®,
Impella CP®, Impella CP® with SmartAssist, Impella
5.0® and Impella LD® are FDA approved heart pumps
used to treat heart attack or cardiomyopathy patients in cardiogenic
shock, and have the unique ability to enable native heart recovery,
allowing patients to return home with their own heart. To learn more
about the Impella platform of heart pumps, including their approved
indications and important safety and risk information associated with
the use of the devices, please visit: www.protectedpci.com.
The ABIOMED logo, ABIOMED, Impella, Impella 2.5, Impella 5.0, Impella
LD, Impella CP, Impella RP, Impella Connect, and Recovering hearts.
Saving lives. are registered trademarks of ABIOMED, Inc. in the U.S. and
in certain foreign countries.
ABOUT ABIOMED
Based in Danvers, Massachusetts, Abiomed, Inc. is a leading provider of
medical devices that provide circulatory support. Our products are
designed to enable the heart to rest by improving blood flow and/or
performing the pumping of the heart. For additional information, please
visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements
regarding development of Abiomed's existing and new products, the
Company's progress toward commercial growth, and future opportunities
and expected regulatory approvals. The Company's actual results may
differ materially from those anticipated in these forward-looking
statements based upon a number of factors, including uncertainties
associated with development, testing and related regulatory approvals,
including the potential for future losses, complex manufacturing, high
quality requirements, dependence on limited sources of supply,
competition, technological change, government regulation, litigation
matters, future capital needs and uncertainty of additional financing,
and other risks and challenges detailed in the Company's filings with
the Securities and Exchange Commission, including the most recently
filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q.
Readers are cautioned not to place undue reliance on any forward-looking
statements, which speak only as of the date of this release. The Company
undertakes no obligation to publicly release the results of any
revisions to these forward-looking statements that may be made to
reflect events or circumstances that occur after the date of this
release or to reflect the occurrence of unanticipated events.

View source version on businesswire.com: https://www.businesswire.com/news/home/20190131005297/en/



